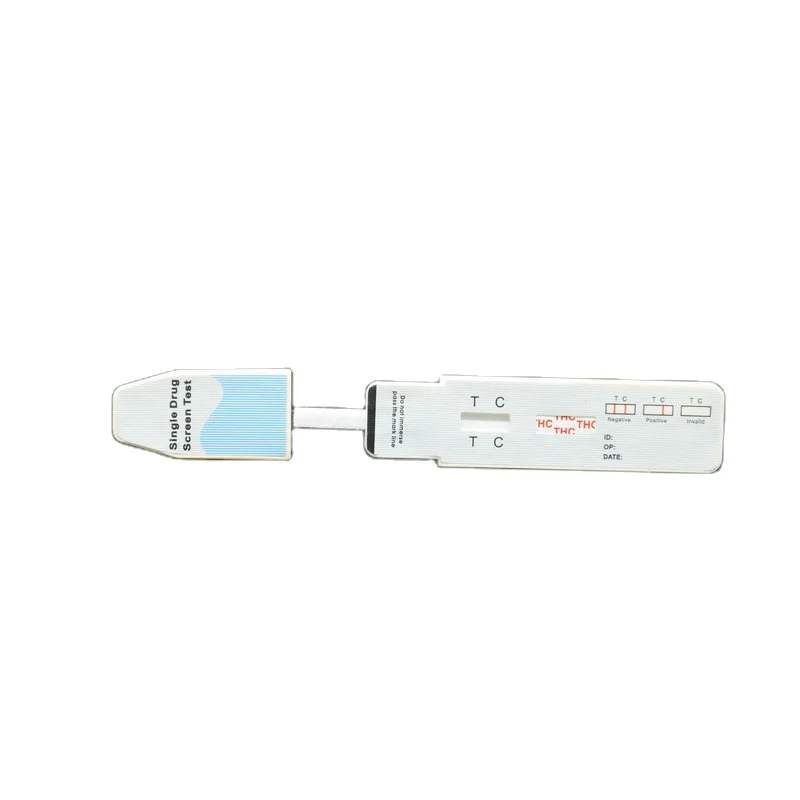
The COT One Step Cotinine Test Device (Urine) is a lateral flow chromatographic immunoassay for the detection of Cotinine in human urine at a cut-off concentration of 200 ng/mL. This test will detect other related compounds, please refer to the Analytical Specificity table in this package insert.
This assay provides only a preliminary analytical test result. A more specific alternate chemical method must be used in order to obtain a confirmed analytical result. Gas chromatography and mass spectrometry (GC/MS) is the preferred confirmatory method.
Clinical consideration and professional judgment should be applied to any drug of abuse test result, particularly when preliminary positive results are used. Cotinine is the first-stage metabolite of nicotine, a toxic alkaloid that produces stimulation of the autonomic ganglia and central nervous system when in humans. Nicotine is a drug to which virtually every member of a tobacco-smoking society is exposed whether through direct contact or second-hand inhalation. In addition to tobacco, nicotine is also commercially available as the active ingredient in smoking replacement therapies such as nicotine gum, transdermal patches and nasal sprays.
In a 24-hour urine, approximately 5% of a nicotine dose is excreted as unchanged drug with 10% as cotinine and 35% as hydroxycotinine; the concentrations of other metabolites are believed to account for less than 5%.1 While cotinine is thought to be an inactive metabolite, it’s elimination profile is more stable than that of nicotine which is largely urine pH dependent. As a result, cotinine is considered a good biological marker for determining nicotine use. The plasma half-life of nicotine is approximately 60 minutes following inhalation or parenteral administration.2 Nicotine and cotinine are rapidly eliminated by the kidney; the window of detection for cotinine in urine at a cutoff level of 200 ng/mL is expected to be up to 2-3 days after nicotine use.
Precautions:
- This kit is for external use only. Do not swallow.
- Discard after first use. The test cannot be used more than once.
- Do not use test kit beyond expiry date.
- Do not use the kit if the pouch is punctured or not well sealed.
- Keep out of the reach of children.
- Do not read after 5 minutes
Name of available drugs to be test list
| Diagnostic Rapid Test Name/Speciments | Format | size | sensitivity |
| AMP One Step Amphetamine Test(Urine) | strip | 3.0mm | 500ng/ml |
| cassette | 5.0mm | ||
| BAR One Step Barbiturates Test(Urine) | strip | 3.0mm | 300ng/ml |
| cassette | 5.0mm | ||
| BZO One Step Benzodiazepines Test(Urine) | strip | 3.0mm | 300ng/ml |
| cassette | 5.0mm | ||
| COC One Step Cocaine Test(Urine) | strip | 3.0mm | 300ng/ml |
| cassette | 5.0mm | ||
| MET One Step Methamphetamine Test(Urine) | strip | 3.0mm | 1000ng/ml |
| cassette | 5.0mm | ||
| MDMA One Step Ecstasy Test (Urine) | strip | 3.0mm | 500ng/ml |
| cassette | 5.0mm | ||
| MOR One Step Morphine Test Strip (Urine) | strip | 3.0mm | 300ng/ml |
| cassette | 5.0mm | ||
| MTD One Step Methadone Test Strip (Urine) | strip | 3.0mm | 300ng/ml |
| cassette | 5.0mm | ||
| OPI One Step Opiate Test Strip (Urine) | strip | 3.0mm | 2000ng/ml |
| cassette | 5.0mm | ||
| PCP One Step Phencyclidine Test (Urine) | strip | 3.0mm | 25ng/ml |
| cassette | 5.0mm | ||
| TCA One Step Tricyclic Antidepressants Test (Urine) | strip | 3.0mm | 1000ng/ml |
| cassette | 5.0mm | ||
| THC One Step Marijuana Test (Urine) | strip | 3.0mm | 50ng/ml |
| cassette | 5.0mm | ||
| Multi-Drug test (Urine) | 2 in 1 test panel | 5.0mm | |
| 3 in 1 test panel | 5.0mm | ||
| 4 in 1 test panel | 5.0mm | ||
| 5 in 1 test panel | 5.0mm | ||
| 6 in 1 test panel | 5.0mm | ||
| 10 in 1 test panel | 5.0mm | ||
| Multi-Drug test cup (Urine) | 5 in 1 test cup | 5.0mm | |
| 6 in 1 test cup | red cap | ||
| blue cap | |||
| Multi-Drug test 6 in 1 (Saliva) | panel | ||
| cup |
How to use the One Step Urine (Multi) Drug (Screen) Test (kit)?
[For Strip]
Remove the test strip from the sealed pouch and use it as soon as possible.
- Immerse the strip vertically into the urine specimen with the arrow end pointing towards the urine. Do not immerse the strip past the Max Line. See the illustration below.
- Remove the strip after 10 seconds and lay the strip flat on a clean, dry, non-absorbent surface, and then begin timing.
- Wait for colored lines to appear. Interpret the test results at 3-5 minutes. Do not read results after 10 minutes

[For Cassette]
- Remove the test cassette from the sealed pouch.
- Hold the dropper vertically and transfer 3 full drops (approx. 100ml) of urine to the specimen well of the test cassette, and then begin timing. See the illustration below.
- Wait for colored lines to appear. Interpret the test results at 3-5 minutes. Do not read results after 10 minutes.

For Dipcard]
- Remove the test card from the sealed pouch and use it as soon as possible.
- Remove the cap from the end of the test card. Immerse the strip(s) of the test card vertically in the urine specimen with arrows pointing toward the urine specimen. Immerse the test card to at least the level of the wavy lines on the strip(s), but not above the arrow(s) on the test card. See the illustration below.
- Remove the test card after 10 seconds and lay the card flat on a non-absorbent surface, and then begin timing.
- Wait for colored lines to appear. Interpret the test results at 3-5 minutes. Do not read results after 10 mins

For Multi-Drug Screen Test Cup]
- Remove the test cup from the sealed pouch and use it as soon as possible.
- Donor provides specimen and secures the cap by pressing down on all three corners.
- On a flat surface, technician pushes key to a fully closed position.
- Peel off the label on the multi-drug test card to view results. The test is read in the reaction well.
- Start the timer and wait for colored lines to appear. Interpret the test results at 3-5 minutes. Results remain stable for up to sixty minutes. See the illustration below. For detailed operation instructions, please refer to the Procedure Card

Results Interpretation: Negative or Positive Indication
Negative:Two lines appear. One red line should be in the control region (C), and another apparent red or pink line adjacent should be in the test region (T). This negative result indicates that the drug concentration is below the detectable level. (*Note: The shade of red in the test line region (T) will vary, but it should be considered negative whenever there is even a faint pink line.)
Positive:One red line appears in the control region (C). No line appears in the test region (T). This positive result indicates that the drug concentration is above the detectable level.
Invalid:Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test using a new test panel. If the problem persists, discontinue using the lot immediately and contact your local distributor








We have more categories for you. lf you can't find the products you want above,just fill in the form and tell us whatproducts you want to import from China.